Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-BOARD PAPER MARCH - 2019-QUESTIONS
- Explain Reimer Tiemann reaction with one example.
Text Solution
|
- How will you convert chlorobenzene into phenol ?
Text Solution
|
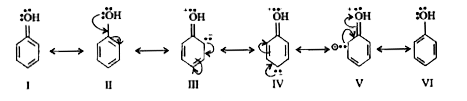
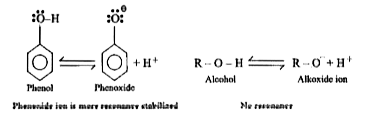
- Why Phenols are more acidic than Alcohol ?
Text Solution
|
- Write two differences between physical adsorption and chemical adsorpt...
Text Solution
|
- Define Emulsion
Text Solution
|
- Write Hell Volhard Zelinsky reaction.
Text Solution
|
- Why are the boiling points of carboxylic acids higher than the corresp...
Text Solution
|
- Explain why aldehydes are more reactive than ketones towards nucleophi...
Text Solution
|
- A face centered cubic element (atomic mass =60) has edge length of 400...
Text Solution
|
- Give one difference between crystalline and amorphous solids.
Text Solution
|
- An element having a density 11.2"g cm"^(-3) forms a fcc lattice with e...
Text Solution
|
- Define unit cell and paramagnetic substance.
Text Solution
|
- Write the following reaction: Wurtz Fittig Reaction
Text Solution
|
- True/False- Stems absorb water and minerals from the soil.
Text Solution
|
- True/False- The plant gets nitrogen from air.
Text Solution
|
- Why solubility of Haloalkanes in water is very low ?
Text Solution
|
- Give one use of freon.
Text Solution
|
- Why nitrogen is important for the plants?
Text Solution
|
- Give the mechanism of substitution nucleophilic bimolecular, SN^2 reac...
Text Solution
|
- Define Optical activity.
Text Solution
|