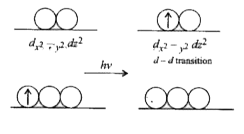Text Solution
Verified by Experts
Topper's Solved these Questions
BOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - C|11 VideosBOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - D|18 VideosBOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - D|18 VideosBOARD PAPER MARCH - 2019
ACCURATE PUBLICATION|Exercise QUESTIONS|54 VideosCHEMICAL KINETICS
ACCURATE PUBLICATION|Exercise TOPIC : CHEMICAL KINETIC|69 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-BOARD PAPER MARCH - 2020-SECTION - B
- Define calcination and roasting.
Text Solution
|
- What are inter halogen compound ? Give preparation of CIF.
Text Solution
|
- Transition metals form mostly coloured compounds.Explain.
Text Solution
|
- Write two difference between double salt and complex compound.
Text Solution
|
- Give IUPAC name of following K2 [ HgCl4]
Text Solution
|
- Give IUPAC name of following K2 [ Ni(CN)4]
Text Solution
|
- Write two differences between hormones and vitamins.
Text Solution
|
- The rate constant for a first order reaction is 80 s^-1. How much time...
Text Solution
|
- First order reaction is found to have rate constant, k= 5.5xx 10^(-14)...
Text Solution
|
- Calculate two third life of first order reaction having K = 5.48 xx 10...
Text Solution
|