Text Solution
Verified by Experts
Topper's Solved these Questions
BOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - D|18 VideosBOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - B|10 VideosBOARD PAPER MARCH - 2019
ACCURATE PUBLICATION|Exercise QUESTIONS|54 VideosCHEMICAL KINETICS
ACCURATE PUBLICATION|Exercise TOPIC : CHEMICAL KINETIC|69 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-BOARD PAPER MARCH - 2020-SECTION - C
- Boiling point of benzene is 353.23 K . When 1 . 80 g of non-volatile ...
Text Solution
|
- Difference between osmosis and diffusion.
Text Solution
|
- Explain Victor Meyer’s test for primary (1^@) alcohol.
Text Solution
|
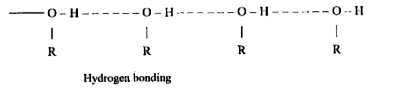
- The Boiling Point of ethers are lower than isomeric alcohols why ?
Text Solution
|
- Write Hell Volhard Zelinsky reaction.
Text Solution
|
- Write Rosenmund reaction.
Text Solution
|
- Explain Clemmensen's reduction.
Text Solution
|
- Write Aldol condensation reaction.
Text Solution
|
- What are biodegradable polymers ? Give example.
Text Solution
|
- Calculate the molar conductance at infinite dilution (lambda^om) of Ca...
Text Solution
|
- What are primary and secondary batteries ?
Text Solution
|