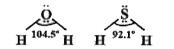Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION - C|6 VideosSOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION -D |16 VideosSOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION -D |16 VideosSOLUTIONS
ACCURATE PUBLICATION|Exercise NUMERICAL QUESTIONS (3 MARKS)|31 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION D|14 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED SAMPLE PAPER, MARCH - 2021 -SECTION - B
- Sodium chloride solution freezes at lower temparature than water but b...
Text Solution
|
- Boiling point of water at 750 mm Hg is 96.63 degree celsius. How many ...
Text Solution
|
- The vapour pressure of pure liquids A and B are 450 and 700 mm Hg at 3...
Text Solution
|
- Define conductivity and molar conductivity for the solution of an elec...
Text Solution
|
- Calculate the half-life of a first order reaction from its rate consta...
Text Solution
|
- A reaction is first order in A and second order in B How is the rate...
Text Solution
|
- Why do noble gases have large atomic size
Text Solution
|
- Which form of sulphur shows paramagnetic behaviour and why ?
Text Solution
|
- Compare and explain bond angles of H2O and H2S
Text Solution
|
- What are interstitial compounds ? Why are such compounds well known fo...
Text Solution
|
- Why are Mn^(2+) compounds more stable than Fe^(2+) compounds towards o...
Text Solution
|
- What is meant by unidentate, didentate and ambidentate ligands ? Give ...
Text Solution
|
- [Fe(CN)6)]^(4-) and [Fe(H2O)6)]^(2+) are of different colours in di...
Text Solution
|
- Explain the factors affecting rate of a reaction.
Text Solution
|