Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-2-SECTION C
- What happens when 1^@, 2^@ and 3^@ alcohols are passed over red hot co...
Text Solution
|
- Write Victor Meyer's test to distinguish between 1^@, 2^@ and 3^@ alc...
Text Solution
|
- A first order reaction is 15% complete in 20 minutes. How long will it...
Text Solution
|
- The reaction 2A+B+C= D +2 E is of first order with respect to A and of...
Text Solution
|
- Give cell is {:([Ni|Ni^(2+) ||Cu^(2+)|Cu]),(" (0.01m) (0.1m)"):} ...
Text Solution
|
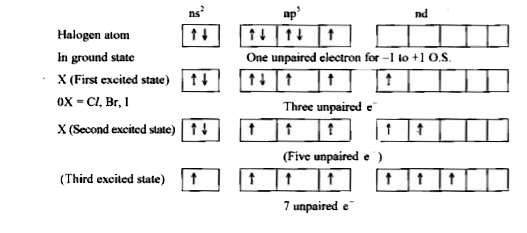
- Why fluorine shows-1 oxidation state only whereas other halogens show ...
Text Solution
|