Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION C|11 VideosSOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION D|12 VideosSOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION A(TRUE/FALSE TYPE QUESTIONS)|5 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION D|11 VideosSURFACE CHEMISTRY
ACCURATE PUBLICATION|Exercise COMPREHENSION QUESTIONS |49 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-3-SECTION B
- Why is Copper considered as transition metal ?
Text Solution
|
- Define Ambident ligands ?
Text Solution
|
- Write the IUPAC name of following : K[Ag (CN)2]
Text Solution
|
- Write the IUPAC name of the following: [Ni(H2O)2(NH3)4]SO4
Text Solution
|
- How will you show that elevation in boiling point is a colligative pro...
Text Solution
|
- What are the units of rate constant for a third order reaction ?
Text Solution
|
- Why are Lanthanides called inner transition metals.
Text Solution
|
- Oxygen gas is inert at room temperature why?
Text Solution
|
- Halogens have maximum negative electron gain enthalpy in the respectiv...
Text Solution
|
- Write two uses of Chlorine.
Text Solution
|
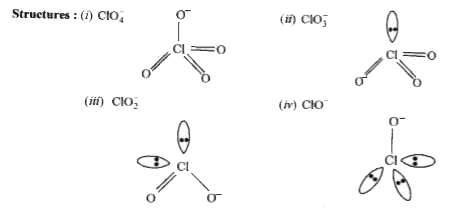
- Arrange the different oxoacids of chlorine in increasing order of acid...
Text Solution
|
- The decomposition of NH3 on platinum surface is zero order reaction. I...
Text Solution
|
- A first order reaction has a specific reaction rate is 10^(-2) sec^(-1...
Text Solution
|
- How many grams of ethylene glycol (molar mass = 62) should be added to...
Text Solution
|
- In winter, the normal temperature in Dharmshala is - 8^(@) C Is a 30%...
Text Solution
|
- What is the difference between e.m.f. and potential diffrence?
Text Solution
|