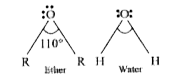Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION D|12 VideosSOLVED MODEL TEST PAPER-3
ACCURATE PUBLICATION|Exercise SECTION B|16 VideosSOLVED MODEL TEST PAPER-2
ACCURATE PUBLICATION|Exercise SECTION D|11 VideosSURFACE CHEMISTRY
ACCURATE PUBLICATION|Exercise COMPREHENSION QUESTIONS |49 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED MODEL TEST PAPER-3-SECTION C
- Discuss the reaction and mechanism of acidic dehydration of ethyl alco...
Text Solution
|
- Phenol are more acidic in nature than alcohol Explain why?
Text Solution
|
- C-O-C bond angle in ethers is higher than H-O-H bond angle in water th...
Text Solution
|
- Ethers possess a dipole moment even if the alkyl groups in the molecul...
Text Solution
|
- Calculate the maximum work that can be obtained from the Daniell cells...
Text Solution
|
- The reaction 2NO2O5to4NO2+O2 forms NO2 at the rate of 0.0072 mol L^(-1...
Text Solution
|
- The reaction 2N2O5to4NO2+O2 forms NO2 at the rate of 0.0072 mol L^(-1)...
Text Solution
|
- The reaction 2NO2O5to4NO2+O2 forms NO2 at the rate of 0.0072 mol L^(-1...
Text Solution
|
- A first order reaction is 20 % complete in 10 minutes. Calculate Spe...
Text Solution
|
- A first order reaction is 20% complete in the 10 minutes. Calculate th...
Text Solution
|
- Why are halogens coloured ?
Text Solution
|