A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SARAS PUBLICATION-THERMODYNAMICS-EXAMPLE
- If the cold junction of a thermocouple is kept at 0^@C and the hot jun...
Text Solution
|
- In thermodynamic processes which of the following statements is not tr...
Text Solution
|
- A black body at 227^@C radiates heat at the rate of 7 cals//cm^-2s^-1A...
Text Solution
|
- If DeltaUand DeltaW represent the increase in internal energy and work...
Text Solution
|
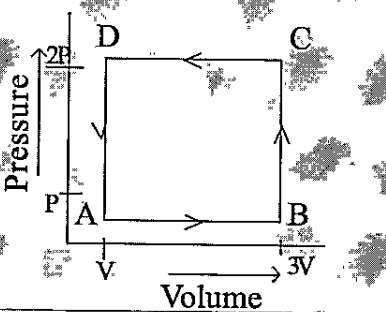
- A thermodynamic system is taken through the cycle ABCD as shown in fig...
Text Solution
|
- The potential energy of a particle in a force field is : U=A/r^2-B/r^1...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- Two pith balls carrying equal charges are suspended from a common poin...
Text Solution
|
- A monatomic gas at a pressure P, having a volume V expands isothermall...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- Two vessels separately contain two ideal gases A and B at the same tem...
Text Solution
|
- An ideal gas compressed to half its initial volume by means of several...
Text Solution
|
- A rod of weight W is supported by two parallel knife edges A and B and...
Text Solution
|
- A gas is compressed isothermally to half its initial volume.The same g...
Text Solution
|
- Therodynamic processes are indicated in the following diagram. .
Text Solution
|