A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
SARAS PUBLICATION-BEHAVIOUR OF PERFECT GAS AND KINETIC THEORY-EXAMPLE
- At 10^@C the value of the density of a fixed mass of an ideal gas div...
Text Solution
|
- The rate of increase of thermo e.m.f with temperature at the neutral t...
Text Solution
|
- Out of the following functions representimg motion of a particle which...
Text Solution
|
- Fusion reaction takes place at high temp .
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state ...
Text Solution
|
- The molar specific heats of an ideal gas at a constant pres...
Text Solution
|
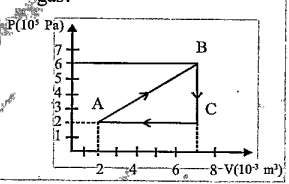
- A gas is taken through the cycle A rarr B rarr C rarr A, as shown. Wh...
Text Solution
|
- In the given (V-T) diagram , what is the relation between P(1) "and" P...
Text Solution
|
- Two carnot engines A and B are operated in series . The engine A rec...
Text Solution
|
- The mean free path of molecules of a gas ,(radius r0 is inverselty pro...
Text Solution
|
- A carnot engine, having efficiency of eta=(1)/(10) as heat engine, is ...
Text Solution
|
- A mass m moves in a circle on a smooth horizontal plane with velocity...
Text Solution
|
- A block of mass 10kg moving in x direction with a constant speed of 10...
Text Solution
|
- 4.0 g of gas occupies 22.4 litres at NTP . The specific heat capacityo...
Text Solution
|
- A series R-C circuit is connected to an alternating voltage source . C...
Text Solution
|
- A particle of mass 10g moves along a circle of radius 6.4 cm with a co...
Text Solution
|
- The molecules of a given mass of a gas have rms velocity of 200ms^(-1)...
Text Solution
|
- A solid sphere of mass m and radius R is rotating about its diameter...
Text Solution
|
- A given sample of an ideal gas occupies a volume V at a pressure P and...
Text Solution
|
- A gas mixture consists of 2 moles of O2 and 4 moles of Ar at temperatu...
Text Solution
|
 .
.