Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-IS MATTER AROUND US PURE-IMPORTANT Q/A
- What is a mixture ?Write two properties of mixture.
Text Solution
|
- What are the types of mixtures ?
Text Solution
|
- What is solution ?
Text Solution
|
- what are alloys?
Text Solution
|
- What do you mean by solvent and solute, Give examples.
Text Solution
|
- Is air a mixture or a compound?
Text Solution
|
- What is saturated solution ?
Text Solution
|
- What is unsaturated solution ?
Text Solution
|
- What do you mean by concentration of a solution ?
Text Solution
|
- What is a suspension ? What are its properties?
Text Solution
|
- What is a colloidal solution ?
Text Solution
|
- What is Tyndall effect?
Text Solution
|
- How can we separate a mixture of two immiscible liquids like kerosene ...
Text Solution
|
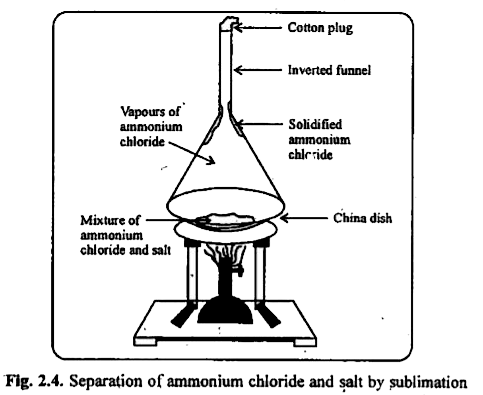
- How will you separate a mixture of Ammonium chloride and common salt ?
Text Solution
|
- How can we separate a mixture of two miscible liquids ?
Text Solution
|
- Distinguish between mixtures and compounds ?
Text Solution
|