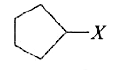
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sodium tertiary butoxide forms ether only with :
Text Solution
|
- Formation of methyl tertiary butyl ether by the reaction of sodium ter...
Text Solution
|
- Formation of methyl tertiary butyl ether by the reaction of sodium ter...
Text Solution
|
- (A) Di-tert-butyl ether cannot be prepared by Williamson's ether synet...
Text Solution
|
- Acetone reacrts with butan-2-ol in prsence of aluminium tertiary butox...
Text Solution
|
- Formation of methyl tertiary butyl ether by the reaction of sodium ter...
Text Solution
|
- Assertion (A) : Reaction between (Me(3)CONa) (sodium ter-butoxide) and...
Text Solution
|
- STATEMENT-1 Reaction between sodium tert butoxide and ethyllodide doe...
Text Solution
|
- STATEMENT-1 : Tert butyl bromide and sodium ethoxide will react to for...
Text Solution
|