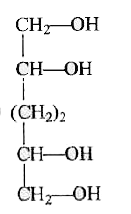
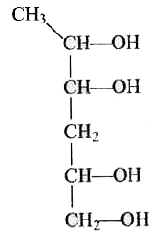
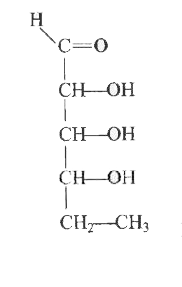
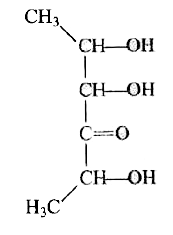
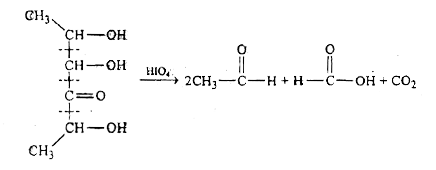A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound A (Molecular formula C(6)H(12)O(4)) on treatment w...
Text Solution
|
- A gaseous alkane on complete combustion gives CO(2) and H(2)O . If the...
Text Solution
|
- Consider the reaction C(6)H(12)O(6) + 12H(2)SO(4) rarr 6CO(2) + 12SO(2...
Text Solution
|
- An organic compound C(4)H(6) on ozonolysis give HCHO, CO(2), CH(3)CHO....
Text Solution
|
- An organic compound with moleular formula, C(2)H(6)O(2) evolves H(2) w...
Text Solution
|
- Calculate the molecular masses of H(2), O(2), Cl(2), CO(2), CH(4), C(2...
Text Solution
|
- Mixture of 1 mole of each CH(4) and C(2)H(6) absorb 5 mole of Cl(2) to...
Text Solution
|
- निम्न यौगिकों के आण्विक द्रव्यमान का परिकलन कीजिएः H(2), O(2), Cl(2)...
Text Solution
|
- Which gaves 2 moles of HCHO. 1 mole of HCOOH and CO(2) by HIO(4) .
Text Solution
|