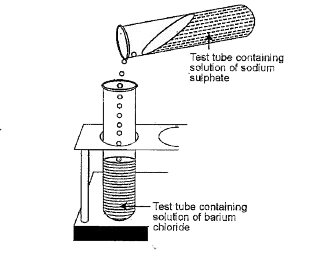
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
NAND LAL PUBLICATION|Exercise ACTIVITY 1.11|1 VideosCHEMICAL REACTIONS AND EQUATIONS
NAND LAL PUBLICATION|Exercise INTEXT QUESTIONS|13 VideosCHEMICAL REACTIONS AND EQUATIONS
NAND LAL PUBLICATION|Exercise ACTIVITY 1.9|5 VideosCARBON AND ITS COMPOUNDS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|3 VideosMETALS AND NON-METALS
NAND LAL PUBLICATION|Exercise ACTIVITY 3.8|3 Videos
Similar Questions
Explore conceptually related problems