Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
NAND LAL PUBLICATION|Exercise EXERCISES|18 VideosCARBON AND ITS COMPOUNDS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|3 VideosCARBON AND ITS COMPOUNDS
NAND LAL PUBLICATION|Exercise ACTIVITY 4.12|6 VideosACIDS, BASES AND SALTS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|8 VideosCHEMICAL REACTIONS AND EQUATIONS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
NAND LAL PUBLICATION-CARBON AND ITS COMPOUNDS-INTEXT QUESTIONS
- What would be the electron dot structure or carbon dioxide which has t...
Text Solution
|
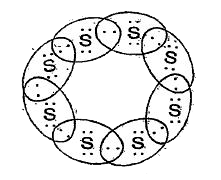
- What would be the electron-dot structure of a molecule of sulphur whic...
Text Solution
|
- How many structural isomers can you draw for pentane?
Text Solution
|
- What are the two properties of carbon which lead to the huge number of...
Text Solution
|
- What will be the formula and electron dot structure of cyclopentane?
Text Solution
|
- Draw the structures for the follow compounds: 2-Pentanone
Text Solution
|
- How would you name the following compounds? CH3-CH2-Br
Text Solution
|
- How would you name the following compound ? H-overset(H)overset(|)C=...
Text Solution
|
- How would you name the following compounds?
Text Solution
|
- Why is the conversion of ethanol to ethanoic acid an oxidation reactio...
Text Solution
|
- A mixture of oxygen and ethyne is brunt for welding. can you tell why ...
Text Solution
|
- How would you distinguish experimentally between an alcohol and a carb...
Text Solution
|
- What are oxidising agents?
Text Solution
|
- Would you be able to check if water is hard using a detergent?
Text Solution
|
- People use variety of methods to wash clothes usually after adding the...
Text Solution
|