Similar Questions
Explore conceptually related problems
Recommended Questions
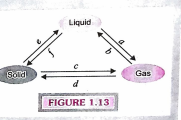
- Study the Fig.1.13. for interconversion of states of matter carefully ...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- How do you define interconversion of states of matter?
Text Solution
|
- Interconversion Of Matter
Text Solution
|
- Interconversion of Matter
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- If we consider the pressure and volume of a gas at a specific tempe...
Text Solution
|
- Explain in detail the effects of changes in temperature and pressure o...
Text Solution
|
- विभिन्न तापमानों पर आयतन के साथ दाब में परिवर्तन को चित्र 5.3 में दिए ...
Text Solution
|