Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I |55 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II|50 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOME BASIC CONCAPTS OF CHEMISTRY -LEVEL-III (Linked Comprehension Type)
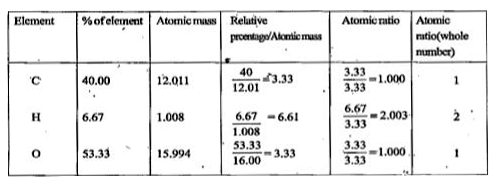
- An organic compound contains 40% carbon, 6.67% hydrogen and 53.33% oxy...
Text Solution
|
- Isotopes are the atoms of same element: they have same atomic number b...
Text Solution
|
- Isotopes are the atoms of same element: they have same atomic number b...
Text Solution
|
- Given that the abundance of isotopes ""^(54)Fe, ""^(56) Fe and ""(57) ...
Text Solution
|
- What is the molarity of "11.2 V" of H(2) O (2) ?
Text Solution
|
- What is the percentage strength (%w/V) of "11.2 V " H (2) O(2) ?
Text Solution
|
- 40 g Ba (MnO (4)) (2) (mol. wt, = 375) sample containing some inert i...
Text Solution
|