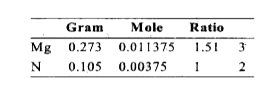A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|14 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Correct Answer Type)|8 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I |55 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOME BASIC CONCAPTS OF CHEMISTRY -LEVEL-II
- 2 g of a mixture of CO and CO2 on reaction with excess I(2)O(5) produc...
Text Solution
|
- If 0.5g of a mixture of two metals A and B with respective equivalent ...
Text Solution
|
- When 0.273g of Mg is heated strongly in a nitrogen (N2) atmosphere, 0....
Text Solution
|
- A spherical ball of radius 7cm contains 56% iron. If density is 1.4g"/...
Text Solution
|
- 6.0 g sample of CaCO(3) reacts with 20 g solution of HCl having 20% b...
Text Solution
|
- If a person inhale 10^(20) oxygen atom per sec, the volume of oxygen g...
Text Solution
|
- The number of O(2) molecules and its volume at S.T.P in 50.6 g of hyd...
Text Solution
|
- 10g of CO on burning in air gives the product which have the number of...
Text Solution
|
- A plant virus is a uniform cylindrical particle of 150A^(@) in diamete...
Text Solution
|
- Haemoglobin contains 0.25% iron by weight. The molecular wt. of haemog...
Text Solution
|
- Copper forms two oxides . For the same amount of copper, twice as much...
Text Solution
|
- 5mL of a gaseous hydrocarbon reacts with 30mL of O2. The resultant gas...
Text Solution
|
- Calculate the mass of calcium oxide required that react with 852 g of ...
Text Solution
|
- Asample of an alloy weighing 0.50 g and containing 90% Ag was dissolve...
Text Solution
|
- 109% labelled oleum has x mole of H(2)SO(4) and y mole of SO3 respecti...
Text Solution
|
- What is the molar mass of a substance, each molecule of which contains...
Text Solution
|
- The molality of an H(2)SO(4) solution is 9. The weight of the solute i...
Text Solution
|
- Calculate the mass of CaO required to remove the hardness of 10^(6) li...
Text Solution
|
- Two litres of NH(3) at 30^(@)C and 0.20 atmosphere is neutralized by ...
Text Solution
|
- How much BaCl2 would be needed to make 250mL of a solution having same...
Text Solution
|