A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Correct Answer Type)|8 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Numerical type)|9 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II|50 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOME BASIC CONCAPTS OF CHEMISTRY -LEVEL-III (Single Correct Answer Type)
- Zinc sulphate contains 22.65% of zinc and 43.9% of water of crystalliz...
Text Solution
|
- 8 litre of H(2) and 6 litre of Cl(2) are allowed to react to maximum...
Text Solution
|
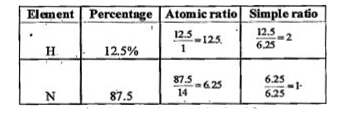
- A gaseous compound of nitrogen and hydrogen contains 12.5% (by mass) o...
Text Solution
|
- 100 mLofa gas at STP was heated with Tin. Tin converted into stannous ...
Text Solution
|
- A mixture of HCOOH and H(2) C(2) O(4) is heated with conc. H(2)SO(4)....
Text Solution
|
- A solid mixture of 5 g of lead nitrate and sodium nitrate was heated b...
Text Solution
|
- 25 mL of a solution of Na(2) CO(3) having a specific gravity of 1.25g...
Text Solution
|
- Calculate the number of millilitres of Ammonia, (aq) solution (d=0.986...
Text Solution
|
- A mineral consists of an equimolar mixture of the carbonates of two bi...
Text Solution
|
- 1M NaOH solution was slowly added into 1000 mL of 183.75 g impure H(2)...
Text Solution
|
- 20 mL of 0.2 M NaOH(aq) solution is mixed with 35 mL of 0.1 M NaOH(aq)...
Text Solution
|
- A 1.0 g sample of a pure organic compound containing chlorine is fused...
Text Solution
|
- A 5.0 g sample containing Na(2) CO(3) and Na(2) SO(4) is dissolved in ...
Text Solution
|
- A metal ‘M' of atomic mass 54.94 has a density of 7.42 g/cm^(3): Calcu...
Text Solution
|