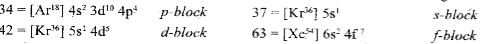Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-I)|50 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-II)|51 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion - Reason Type)|20 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosENVIRONMENTAL CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS LEVEL-II (ASSERTION-REASON TYPE)|10 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTIONS
- Find the blocks to which elements having atomic numbers 34, 37, 42 , 6...
Text Solution
|
- Predict the period, group and block to which elements with atomic numb...
Text Solution
|
- Name the element with atomic number 119.
Text Solution
|
- The electronegativities of carbon and chlorine are 2.5 and 3.00 respec...
Text Solution
|
- The electron gain ethalpy of chlorine is -349kJ mol^(-1) . Calculate t...
Text Solution
|
- Calculate the N-O bond distance if covalent radii of nitrogen and oxyg...
Text Solution
|
- What is the effective nuclear charge, felt by a 1s electron of the hel...
Text Solution
|
- Calculate the Z(eff) felt by a 2p electron of the nitrogen atom.
Text Solution
|
- Calculate the Z(eff) at the periphery of the chromium atom.
Text Solution
|
- Calculate the electronegativity of carbon from the following data: E(H...
Text Solution
|
- Calculate the electronegativity of silicon ( covalent radius= 1.175 Å)
Text Solution
|