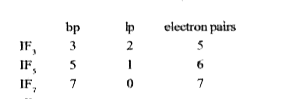A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II|100 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-I
- Which of the following statements regarding I(3)^(-) is not correct?
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- The number of pairs of electrons around I in IF3, IF5 and IF7, respect...
Text Solution
|
- Which of the following species has linear structure?
Text Solution
|
- The energy of sigma 2s is greater than sigma^("*")1s Is orbital becaus...
Text Solution
|
- The hybridization of chlorine orbitals in the compound ClF3 is
Text Solution
|
- Which of the following ions does not involve sp^3 hybridization?
Text Solution
|
- The hybridization of Xe in XeOF4 is
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- The number of bonding and antibonding electrons in O2^(2-) ion, respec...
Text Solution
|
- Which of the following pairs of species has identical value of bond or...
Text Solution
|
- Which of the following is expected to be diamagnetic? H2^(+), B2, C...
Text Solution
|
- Which one of the following species is expected to have bond order 1//2...
Text Solution
|
- Which one of the following species will have maximum bond dissociation...
Text Solution
|
- Which of the following statements regarding carbon monoxide is correct...
Text Solution
|
- In which one of the following compounds does hydrogen bonding occur?
Text Solution
|
- A covalent bond is most likely to be formed between two elements which
Text Solution
|
- Which of the following orbitals of a diatomic molecule AB oriented alo...
Text Solution
|
- The dipole moment of hydrogen chloride (bond distance: 127pm) is 1.03 ...
Text Solution
|
- Which of the following unit conversion of dipole moment is correct? :...
Text Solution
|