A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II|100 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-I
- The dipole moment of hydrogen chloride (bond distance: 127pm) is 1.03 ...
Text Solution
|
- Which of the following unit conversion of dipole moment is correct? :...
Text Solution
|
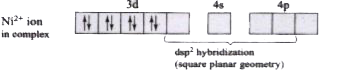
- The geometry of CN groups around Ni in [Ni(CN)4]^(2-) is : tetrahedr...
Text Solution
|
- The sp^3d^2 hybridization of central atom of a molecule would lead to:...
Text Solution
|
- The bond angle in PH3 is expected to be : 90^@, 105^@, 109^@, 120^...
Text Solution
|
- The bond order of CO and NO is : 3 and 2, 3 and 2.5, 3 and 1.3, 3...
Text Solution
|
- Which of the following statements regarding valence bond method is not...
Text Solution
|
- Which of the following molecules is paramagnetic? C2, N2, O2, F2
Text Solution
|
- The bond order of O2 molecule is : 1, 2, 2.5, 3
Text Solution
|
- Which of the following order of energies of molecular orbitals of N2 i...
Text Solution
|
- Which of the following orders regarding the bond order is correct? : ...
Text Solution
|
- Which of the following species has the shortest bond length? N2^(+), ...
Text Solution
|
- Which of the following is the most ionic? : P4O(10), MnO, CrO3, M...
Text Solution
|
- AlCl3 is covalent while AlF3 is ionic. This can be justified on the ba...
Text Solution
|
- In PO4^(3-), P-O bond order is : 1.25, 2, -0.75, -3
Text Solution
|
- In which of the following species the bonds are non-directional? : N...
Text Solution
|
- There is no S - S bond in : S2O6^(2-), S4O6^(2-), S2O3^(2-), S2O7...
Text Solution
|
- Among the following the electron-deficient compound is
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in: The molecula...
Text Solution
|
- Which of the following is a positive overlap which leads to bonding?
Text Solution
|