Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Matching Column Type )|5 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Satement Type )|5 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Correct Answer Type )|12 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-III ( Numerical Type )
- Among the triatomic molecules/ions, BeCl2, N3^- , N2O, NO2^+ , O3, SC...
Text Solution
|
- A sigma- bonded molecule MX3 is T-shaped. The number of non-bonding p...
Text Solution
|
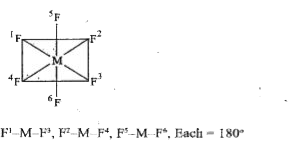
- In a regular octahedral molecule SF6 the number of F-S-F bonds at 180...
Text Solution
|
- The maximum number of atoms lying the same plane in B2 H6 is .........
Text Solution
|
- A slightly polar molecule AB has dipole moment of 0.24 D. If bond-leng...
Text Solution
|
- How many of the following compounds have sp^3 hybridization? i. ...
Text Solution
|