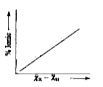
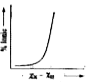
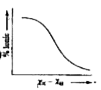
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Satement Type )|5 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-III ( Linked Comprehension Type )
- In an ionic bond, the cation tends to polarize the electron cloud of t...
Text Solution
|
- Among the following which will have the lowest melting point and the h...
Text Solution
|
- Arrange the following species in decreasing order of polarising powers...
Text Solution
|
- Percent ionic characters for HF, HBr and HI follow the order.
Text Solution
|
- The percent ionic character of a polar bond may be computed from the e...
Text Solution
|
- The percent ionic character of a polar bond may be computed from the e...
Text Solution
|
- On the basis of M.O. theory which one of the following species does no...
Text Solution
|
- Which one of the following is paramagnetic and has bond order 1/2?
Text Solution
|
- During change of NO^+ to NO, the electron is added to:
Text Solution
|
- In which of the following molecules is the bond angle largest?
Text Solution
|
- The shape of a molecule is determined by electron-pair repulsions in t...
Text Solution
|
- Which of the following species will have the lone pair effects cancell...
Text Solution
|