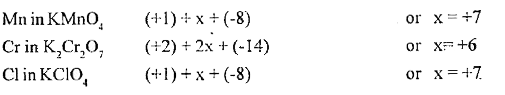Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -I|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -II|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION-REASON TYPE)|20 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-QUESTION
- Find out the oxidation number of C " in " CO2
Text Solution
|
- Find out the oxidation number of Cr " in" Cr2 O(7)^(2-)
Text Solution
|
- Find out the oxidation number of Pb " in " Pb3 O4
Text Solution
|
- What is the oxidation number of iodine in the following compound: KI
Text Solution
|
- What is the oxidation number of iodine in the following compound: I...
Text Solution
|
- What is the oxidation number of iodine in the following compounds: I...
Text Solution
|
- What is the oxidation number of iodine in the following compounds: I...
Text Solution
|
- What is the oxidation number of iodine in the following compounds: ...
Text Solution
|
- Find the oxidation number of Mn, Cr and Cl in KMnO4, K2Cr2O7 and KClO4...
Text Solution
|
- Valency of C is 4 but its O.N. vary from -4 to +4. is it true or false...
Text Solution
|
- Write the net ionic equation for the reaction of potassium dichromate ...
Text Solution
|
- Permanganate ion reacts with bromide ion in basic medium to give manga...
Text Solution
|
- Write the balanced ionic equation for the reaction of zinc with conc. ...
Text Solution
|
- Write ionic equation for the reaction between Cr(2)O(7)^(2-) ions and ...
Text Solution
|
- Give the balanced ionic equation for the oxidation of I^(-) by MnO4^(-...
Text Solution
|