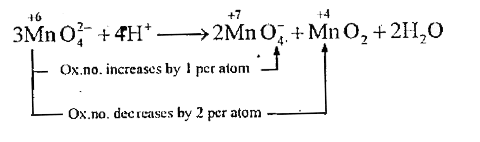A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -II|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise QUESTION|15 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -I
- In which of the following compounds, the oxidation number of iodine is...
Text Solution
|
- Which of the following processes does not involve oxidation of iron?
Text Solution
|
- MnO4^(2-) undergoes disproportionation reaction in acidic medium but M...
Text Solution
|
- In half reaction: S2O3^(2-) to S4O6^(2-) .The number of electrons that...
Text Solution
|
- Which of the following chemical reactions depicts the oxidizing behavi...
Text Solution
|
- In which of the following changes, there is transfer of five electrons...
Text Solution
|
- The following reaction describes the production of metallic iron: 2Fe2...
Text Solution
|
- The correct order of compounds in the decreasing order based on the ox...
Text Solution
|
- Which products are expected from the disproportionation of hypochlorou...
Text Solution
|
- The equivalent mass of oxidising agent in the following reaction is, S...
Text Solution
|
- Complete the balancing of the following half reaction, taking place in...
Text Solution
|
- Consider the metals: Mn, Mg, Zn, Ag, Cu. Based on their reactivity ord...
Text Solution
|
- SO2 under atmospheric condition changes to SOx^(2-) .If oxidation numb...
Text Solution
|
- Equivalent weight of H3PO2 (molecular weight=M) when it disproportiona...
Text Solution
|
- In the reaction, C4H(10(l)) + Cr(2)O(7(aq))^(2-) + H((aq))^(+) to H6C4...
Text Solution
|
- Number of moles of K2Cr2O7, reduced by one mole of Sn^(2+) ions is:
Text Solution
|
- In the alkaline medium, the colour of potassium dichromate solution ch...
Text Solution
|
- A compound contains atoms of three elements A, B, and C. If the oxidat...
Text Solution
|
- H2O2 acts as a reducing agent in
Text Solution
|
- Oxidation number of S in H2SO5 is
Text Solution
|