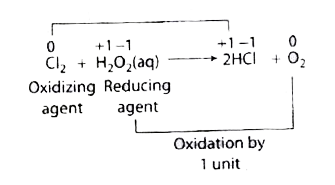A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -II|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise QUESTION|15 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -I
- In the alkaline medium, the colour of potassium dichromate solution ch...
Text Solution
|
- A compound contains atoms of three elements A, B, and C. If the oxidat...
Text Solution
|
- H2O2 acts as a reducing agent in
Text Solution
|
- Oxidation number of S in H2SO5 is
Text Solution
|
- Which one is not correct about the change given below? K4[Fe(CN)6] ove...
Text Solution
|
- Which of the following isintermolecular redox reaction?
Text Solution
|
- The oxidation number of sulphur in S8, S2F2 and H2S are:
Text Solution
|
- In a reaction, 4 mole of electrons are transferred to 1 mole of HNO3, ...
Text Solution
|
- The colour of K2Cr2O7changes from red-orange to lemon yellow on treatm...
Text Solution
|
- One mole of hydrazine (N(2)H(4)) loses 10 moles of electrons in a reac...
Text Solution
|
- For the redox reaction, MnO4^(-) + C2O4^(2-) + H^(+) to Mn^(2+) + CO2 ...
Text Solution
|
- The oxidation state of chromium in the final product formed in the rea...
Text Solution
|
- Amongst the following, identify the species with an atom in +6 oxidati...
Text Solution
|
- The reaction of white phosphorous with aqueous NaOH gives phsophine al...
Text Solution
|
- Hydrogen peroxide in its reaction with KIO4 and NH2OH respectively, is...
Text Solution
|
- Arrange the following in the increasing order of oxidation state of Mn...
Text Solution
|
- Which of the following has least oxidation state of Fe?
Text Solution
|
- In the reaction, 8Al + 3Fe3O4 to 4Al2O3 + 9Fe, the number of electrons...
Text Solution
|
- In the balanced chemical reaction, IO3^(-) + aI^(-) + bH^(+) to cH2O +...
Text Solution
|
- In alkaline medium ClO2 oxidises H2O2 to O2 and itself gets reduced...
Text Solution
|