A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -I|50 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -II
- For the redox reaction, Zn + NO3^(-) to Zn^(2+) + NH4^(+) in basic me...
Text Solution
|
- A solution containing Cu^(+2), C2 O(4)^(-2) ions: The solution requi...
Text Solution
|
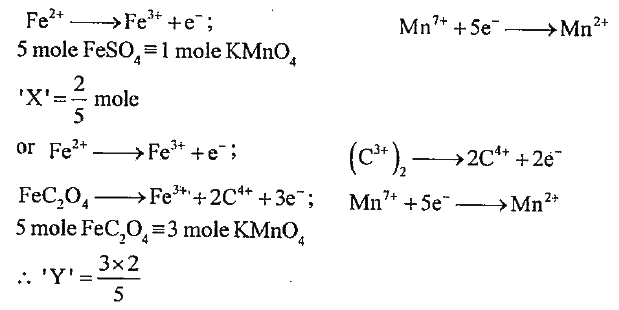
- 2 mole of FeSO4 in acidic medium are oxidised by 'X' mole of KMnO4 whe...
Text Solution
|
- A solution of 0.1 M KMnO4 is used for the reaction S2O3^(-2) + 2MnO4^(...
Text Solution
|
- C2O(4)^2- + MnO4^(-) overset(H^+)(rarr)Mn^(2+) + CO2 The number of H...
Text Solution
|
- In the standardization of Na2S2O3 using K2Cr2O7 by iodometry, the equi...
Text Solution
|
- In redox reaction, Ba(MnO4 )2 oxidizes K4[ Fe(CN)6 ] into Fe^(3+), CO(...
Text Solution
|
- When I^(-) is oxidised by MnO(4)^(-) in alkaline medium, I^(-) convert...
Text Solution
|
- Consider a titration of potassium dichromate solution with acidified M...
Text Solution
|
- Amount of oxalic acid present in a solution can be determined by its t...
Text Solution
|
- In the oxidation of sulphite to sulphate using permanganate, the numbe...
Text Solution
|
- Which ordering of compounds is according to the decreasing order of th...
Text Solution
|
- In which of the following reactions H2O2 acts as a reducing agent? i...
Text Solution
|
- The oxidation state of nitrogen is correctly given for
Text Solution
|
- In the reaction, 3Br2 + 6CO3^(2-) + 3H2O to 5Br^(Theta) + BrO3^(Theta)...
Text Solution
|
- Which of the following is not a disproportionation reaction? .
Text Solution
|
- When KMnO4 acts as an oxidising agent and ultimately form MnO4^(2-), M...
Text Solution
|
- The oxidation states of the most electronegative element in the produc...
Text Solution
|
- In the compound Y Ba2Cu3O7 which shows super conductivity, what is the...
Text Solution
|
- Oxidation states of the metal in the minerals haematite and magnetite,...
Text Solution
|