A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -III|50 VideosREDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -I|50 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -II
- In the reaction, 3Br2 + 6CO3^(2-) + 3H2O to 5Br^(Theta) + BrO3^(Theta)...
Text Solution
|
- Which of the following is not a disproportionation reaction? .
Text Solution
|
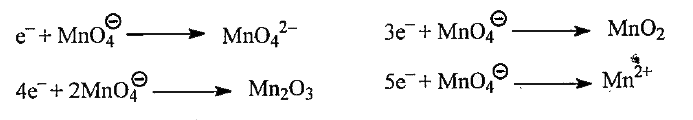
- When KMnO4 acts as an oxidising agent and ultimately form MnO4^(2-), M...
Text Solution
|
- The oxidation states of the most electronegative element in the produc...
Text Solution
|
- In the compound Y Ba2Cu3O7 which shows super conductivity, what is the...
Text Solution
|
- Oxidation states of the metal in the minerals haematite and magnetite,...
Text Solution
|
- Which of the following molecules can acts as oxidising as well as redu...
Text Solution
|
- In balancing the half reaction, CN^(Theta) to CNO^(Theta) (skeletal) ...
Text Solution
|
- In which of the following pairs of compounds, nitrogen has maximum pos...
Text Solution
|
- In the reaction C2H5OH + xI2 + 6OH^(-) to CHI3 + HCO2^(-) + yI^(-) + 5...
Text Solution
|
- The stoichiometric numbers appearing from left to right in the reactio...
Text Solution
|
- Oxidation number of oxygen in potassium superoxide is
Text Solution
|
- Given are the nickel compounds Ni(CO4), K2[NiF6) and K2 [Ni(CN)4]. The...
Text Solution
|
- Which one of the following equations represents a redox reaction?
Text Solution
|
- In the reaction Na2S2O3 + I2 to Na2S4O6 + NaI(not balanced), which of ...
Text Solution
|
- How many electrons are involved in the following redox reaction? Cr2...
Text Solution
|
- Consider the chemical change which is occuring in basic medium : CIO3^...
Text Solution
|
- Choose the incorrect statement in the following:
Text Solution
|
- How many moles of KMnO4 are required in the acidic medium for complete...
Text Solution
|
- When copper is treated with a certain concentration of nitric acid, ni...
Text Solution
|