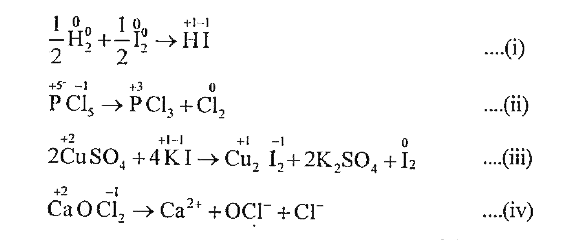
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -II|50 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -III
- Which of the following reactions should be balanced in basic medium?
Text Solution
|
- For the reaction: I^(-) +CIO(3)^(-) +H2 SO4 to Cl^(-) +HSO(4)^(-...
Text Solution
|
- Which of the following are redox reactions?
Text Solution
|
- H2 C2 O4 and NaHC2 O4 behave as.acids as well as reducing agents. Whi...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- When the following half-reaction is balanced: CN^(-) to CNO^(-) Which...
Text Solution
|
- When the following redox reaction is balanced in basic medium, MnO(4)^...
Text Solution
|
- Consider the following redox reaction:KMnO4 + Na2 S2 O3 +H^(+) to Mn^...
Text Solution
|
- Which of the following can be oxidized further with a strong oxidising...
Text Solution
|
- Consider the following reaction: CuSO4 +KI+H^(+) Cul(s)+I2 +K2 SO4 Wh...
Text Solution
|
- Which of the following are disproportionation reaction:
Text Solution
|
- For the reaction I^(-) +CIO(3)^(-) +H2 SO4 to Cl^(-) +HSO(4)^(...
Text Solution
|
- If 2.68 xx 10^(-3) mol of a solution containing an ion A^(n+) requires...
Text Solution
|
- The brown ring complex compound is formulated as [Fe(H2 O)5 NO]SO4 The...
Text Solution
|
- In the following reaction: XZn + yHNO3 (dil) to aZn(NO3)2 + bH2O+cNH...
Text Solution
|
- The difference in the oxidation numbers of two types of sulphur atoms ...
Text Solution
|
- The number of moles of KmnO(4) reduced by 1 mol of Kl in alkaline medi...
Text Solution
|
- In the following reaction, M^(x+)+MnO4^(-) to MO3^(-) +Mn^(2+)+1/2 O...
Text Solution
|
- The number of electrons lost in the following change is: Fe+H2 O to Fe...
Text Solution
|
- Match the reactions in column I with their respective characteristics ...
Text Solution
|