Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
BRILLIANT PUBLICATION|Exercise LEVEL -II|50 VideosPURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL- III (Linked Comprehension Tyne (Paragraph I))|12 VideosS-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-REDOX REACTIONS-LEVEL -III
- In the following reaction, M^(x+)+MnO4^(-) to MO3^(-) +Mn^(2+)+1/2 O...
Text Solution
|
- The number of electrons lost in the following change is: Fe+H2 O to Fe...
Text Solution
|
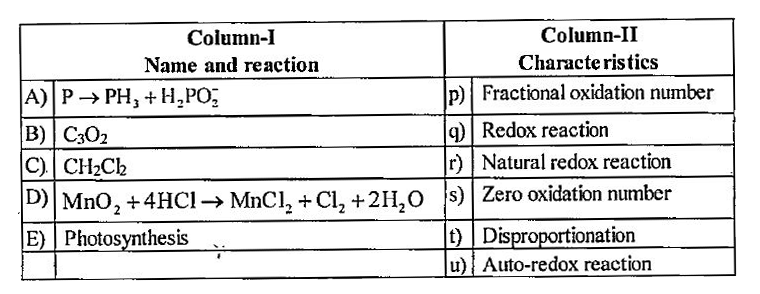
- Match the reactions in column I with their respective characteristics ...
Text Solution
|
- Match the reactions in column I with the nature of the reactions/type ...
Text Solution
|
- Match the compound with the average oxidation state of Fe.
Text Solution
|
- Match the reaction with its type.
Text Solution
|
- Match the reactions given in column I with their respective oxidant/re...
Text Solution
|
- Assertion : Conversion of potassium ferrocyanide to potassium ferricya...
Text Solution
|
- Assertion : In the reaction between potassium permanganate and potassi...
Text Solution
|
- Assertion : Displacement reactions of chlorine, bromine and iodine usi...
Text Solution
|
- Statement 1 : In CIF3, chlorine has the oxidation number-1. Statement ...
Text Solution
|
- Assertion : The two Fe atoms in Fe3O4, have different oxidation number...
Text Solution
|
- The salt of an alkali metal gives violet colour in the flame test. Its...
Text Solution
|
- Which of the following oxides of carbon has fractional oxidation state...
Text Solution
|
- Which of the following compounds of carbon has highest oxidation state...
Text Solution
|
- Oxidation state of carbon in diamond is:
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Which of the following statements is wrong?
Text Solution
|
- Which one of the compound cannot decolourize an acidified solution of ...
Text Solution
|