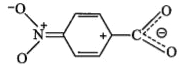
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID & THEIR DERIVATIVES
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|10 VideosCARBOXYLIC ACID & THEIR DERIVATIVES
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Correct Answer Type)|10 VideosCARBOXYLIC ACID & THEIR DERIVATIVES
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|12 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE QUESTIONS)|6 VideosCARBOXYLIC ACIDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion -Reason)|4 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CARBOXYLIC ACID & THEIR DERIVATIVES-LEVEL-II
- In a set of reactions, acetic acid gives the following product 'D'CH(3...
Text Solution
|
- (A), (B) and (C ) are three dicarboxylic acids such that: (A) overse...
Text Solution
|
- HCOOH overset("Conc."H(2)SO(4))to X+Y. Products X and Y are respective...
Text Solution
|
- CH(3)CO(2)C(2)H(5) on reaction with sodium ethoxide in ethanol gives (...
Text Solution
|
- End product of this conversion CH(3)-overset(O)overset(||)C-CH(2)-CH(2...
Text Solution
|
- When acetic acid reacts with ketene, product formed is:
Text Solution
|
- Which of the following compound would be expected to decarboxylate whe...
Text Solution
|
- , A is ?
Text Solution
|
- End product of the following sequence of reaction is:
Text Solution
|
- On subjecting mesityl oxide to the iodoform reaction, one of the produ...
Text Solution
|
- The ease of alkaline hydrolysis is more for:
Text Solution
|
- Which of the following does not form benzoic acid on oxidation with KM...
Text Solution
|
- p-Nitrobenzoic acid is a stronger acid than benzoic acid. This is due ...
Text Solution
|
- The reagents used in the reduction reactions i)CH(3)COC(6)H(4)COOH ...
Text Solution
|
- The intermediate formed when CH(3)COOH react with CH(3)CH(2)OH in pres...
Text Solution
|
- The product of the reaction shown below is,
Text Solution
|
- The compound formed by Claisen condensation of
Text Solution
|
- Which of the following orders is true regarding the boiling point of o...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Which of the following statements is correct?
Text Solution
|