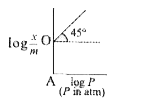A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A graph plotted log (x)/(m) vs. log P is shown in figure given below ...
Text Solution
|
- When a graph is plotted between log x//m and log p, it is striaght lin...
Text Solution
|
- When a graph is plotted between log x/m and log p, it is straight lin...
Text Solution
|
- Graph between log((x)/(m)) and log P is straight line at angle of 45^(...
Text Solution
|
- Graph between log(x/m) vs logP has a slope=2 and intercept = 0.477. Fi...
Text Solution
|
- The mass of gas adsorbed , x , per unit mass of adsorbate , m , was me...
Text Solution
|
- For a adsorption of gas on solid surface, the plots of log x/m vs. log...
Text Solution
|
- In an adsorption experiment a graph between log x/m vs. log p is found...
Text Solution
|
- Graph between "log"(x/m) and log p is a st. line at angle 45^@ with in...
Text Solution
|