A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
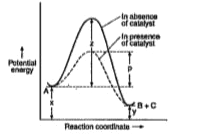
- For the reaction (A rarr B+C) , the energy profile diagram is given i...
Text Solution
|
- Given the following graph. (a) Calculate Delta H for the reaction an...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- At 227^(@)C , the presence of catalyst causes the activation energy of...
Text Solution
|
- Adjoining diagram, represents the energy profile for the reaction : A ...
Text Solution
|
- From the given figure: i) Calculate DeltaE for the reaction and ene...
Text Solution
|
- The negative catalyst decreases the activation energy of a reaction.
Text Solution
|
- Assertion: A catalyst increases the rate of a reaction. Reason: In pre...
Text Solution
|
- Assertion. A catalyst increases the rate of a reaction. Reason. In pre...
Text Solution
|