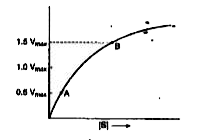
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ezyme [E] is combined with the substrate [S] as follows : E + S ove...
Text Solution
|
- For a first order reaction, the rate of the reaction doubles as the co...
Text Solution
|
- The rates of many substrate reaction catalyzed by enzymes vary with ti...
Text Solution
|
- An enzyme [E] is combined with the substrate [S] as follows: E+S under...
Text Solution
|
- According to the Lock and Key model, the mechanism of an enzyme cataly...
Text Solution
|
- For the reaction : 2A + B rarr C + D, measurement of the rate of the r...
Text Solution
|
- For the reaction , N2O4(g)underset(K2)overset(K1)hArr2NO2(g), the rat...
Text Solution
|
- Consider the reaction mechanism: A(2) overset(k(eq))hArr 2A ("fast")...
Text Solution
|
- अभिक्रिया योजना A overset(k1)toB overset(k2)toC के लिए यदि B के बन...
Text Solution
|