A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
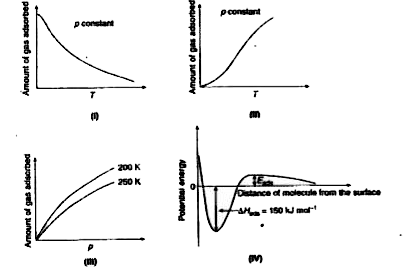
- The given graphs/data I, II, III and IV represent general trends obser...
Text Solution
|
- The given graphs//data I, II, II and IV pepresent general terends obse...
Text Solution
|
- X=amount of gas adsorbed P=Pressure T=Temperature L=Physisorption M=Ch...
Text Solution
|
- The given graph/data I,II,III and IV represent general trends observed...
Text Solution
|
- Write the differences between physisorption and chemisorption with res...
Text Solution
|
- The given graph/data I, II, III and IV represent general trends observ...
Text Solution
|
- दिए हुए I,II,III और IV रेखाचित्र, मंद तापक्रम व दाब पर, विभिन्न भौतिक ...
Text Solution
|
- The given I, II, III and IV diagrams show the general trend of various...
Text Solution
|
- Write the differences between physisorption and chemisorption with res...
Text Solution
|