A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
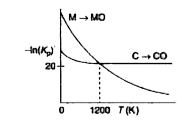
- The plot shows the variation of - In Kp versus temperature for the two...
Text Solution
|
- What is the ratio Kc//Kp for the following reaction at 723^@C ? O2(g) ...
Text Solution
|
- Based on the following thermochemical equations, H2O(g)+C(s) rarr C...
Text Solution
|
- The DeltaH values for reactions of , C(s) + 1/2 O2 (g) to CO(g) D...
Text Solution
|
- 1/2 M2O (s) to M (s) + 1/4 O2(g), DeltaH = 90 kJ mol^(-1) When a sampl...
Text Solution
|
- What conclusion can be drawn from the following reactions about the na...
Text Solution
|
- The plot shows the variation of -InK(p) versus temperature for the two...
Text Solution
|
- Find out Kp//Kc for: CO(g)+frac 1/2 O2 (g) overset (rarr)(larr) CO2 (g...
Text Solution
|
- If C(s) + O2(g) rarr CO2(g), Delta H = X and CO(g) + 1/2 O2(g)rarr CO2...
Text Solution
|