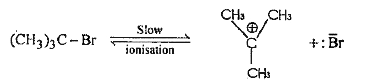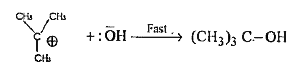Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-HALOALKANES AND HALOARENES-QUESTIONS
- How is DDT prepared from chlorobenzene ? Give the chemical equation on...
Text Solution
|
- Give the reaction chloroform with alcoholic KOH.
Text Solution
|
- Explain with example S(N^1) mechanism.
Text Solution
|
- How tert-butyl bromide reacts with aqueous KOH ? Give the mechanism an...
Text Solution
|
- Explain SN^2 reactions. What is the preference order of different alky...
Text Solution
|
- How tert-butyl bromide reacts with aqueous KOH ? Give the mechanism an...
Text Solution
|
- Give the mechanism of substitution nucleophilic bimolecular, SN^2 reac...
Text Solution
|
- How tert-butyl bromide reacts with aqueous KOH ? Give the mechanism an...
Text Solution
|
- How does chlorobenzene reacts with the following: Sodium
Text Solution
|
- How does chlorobenzene reacts with the following: H2 SO4
Text Solution
|
- How does chlorobenzene react with : Mg.
Text Solution
|
- How does chlorobenzene reacts with the following: KCl2
Text Solution
|
- How does chlorobenzene react with: Lithium
Text Solution
|
- How does chlorobenzene react with: HNO3
Text Solution
|
- What happens when : Chlorobenzene is treated with CH3Cl in the prese...
Text Solution
|
- Explain the following reactions : Nitration of haloarenes.
Text Solution
|
- How does chlorobenzene react with sodium in the presence of ether ? Wh...
Text Solution
|
- How does chlorobenzene react with : Mg.
Text Solution
|
- How does chlorobenzene react with : Cl2.
Text Solution
|
- How does iodobenzene react with copper powder in a sealed tube ? What ...
Text Solution
|