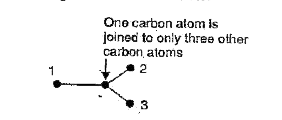Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK 19 (MULTIPLE CHOICE QUESTIONS )|20 VideosCARBON AND ITS COMPOUNDS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET-19 (FILL IN THE BLANKS TO MAKE CORRECT STATEMENTS:)|10 VideosCARBON AND ITS COMPOUNDS
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK 19 |3 VideosATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET -20|23 VideosCOAL AND PETROLEUM
S CHAND IIT JEE FOUNDATION|Exercise Self Assessment Sheet-16 |10 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-CARBON AND ITS COMPOUNDS -QUESTION BANK 19 (NAME THE FOLLOWING )
- Does not have a fixed crystalline shape
Text Solution
|
- what is produced after destructive distillation of coal.
Text Solution
|
- what is an essential constituent of all organic substances.
Text Solution
|
- This is a crystalline form of carbon which has football shaped spheric...
Text Solution
|
- The allotrope of carbon used for making the black cores of pencils.
Text Solution
|
- Allotrope of carbon used in cutting glass and rock drilling.
Text Solution
|
- Amorphous form of carbon used in making shoepolish.
Text Solution
|
- Gas generated in fire extinguishers which is responsible for controlli...
Text Solution
|
- Why should soda-acid and foam-type extinguishers not be used in fighti...
Text Solution
|
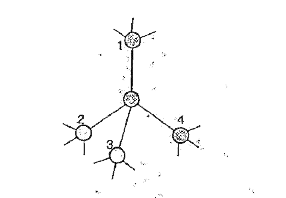
- Why is diamond hard while graphite is soft?
Text Solution
|
- Why is graphite a good conductor of electricity but diamond is not?
Text Solution
|
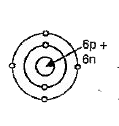
- An element belonging to group 14 of the periodic table has two common ...
Text Solution
|
- A boy sharpens a pencil at both the ends and then uses its back ends t...
Text Solution
|
- A colourless gas is produced on heating strongly a piece of black clec...
Text Solution
|
- Why can inhaling of carbon monoxide become fatal?
Text Solution
|
- Why is it dangerous to sleep in a closed room with a coal fire burning...
Text Solution
|
- How does carbon dioxide help to extinguish fires?
Text Solution
|
- What are the different products formed when methane and chlorine are m...
Text Solution
|