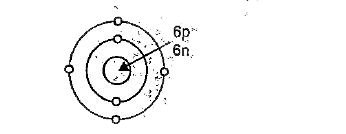
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-CARBON AND ITS COMPOUNDS -SELF ASSESSMENT SHEET-19
- The tip of the lead pencil is made of
Text Solution
|
- What is dry ice?
Text Solution
|
- Why is carbon tetravalent?
Text Solution
|
- What are substitution reactions? Illustrate with an example.
Text Solution
|
- What are hydrocarbons? Give examples
Text Solution
|
- Name the main constituent of natural gas
Text Solution
|
- Describe the structure of diamond
Text Solution
|
- Name the products formed when: wood is burnt in the absence of air
Text Solution
|
- Name the products formed when: bone is heated in the absence of ai...
Text Solution
|
- What is formed when steam is passed over red hot coke?
Text Solution
|
- In diamond, the number of free electrons are:
Text Solution
|
- Which amongst the following is not free state of carbon?
Text Solution
|
- Carbon exists in the atmosphere in the from of
Text Solution
|
- Which of the following statements are usually correct for carbon compo...
Text Solution
|
- Buckminster fullerene is an allotropic form of
Text Solution
|
- which is the same for graphite and diamond:
Text Solution
|
- Inert form of carbon is
Text Solution
|
- Graphite is a good conductor of electricity due to
Text Solution
|
- Diamond is an example of
Text Solution
|
- Which of the following statements are true regarding diamond?
Text Solution
|