Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-20 ( Answer the following questions ) |4 VideosATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET -20|23 VideosATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-20 (FILL IN THE BLANKS WITH APPROPRIATE WORDS TO MAKE CORRECT STATEMENTS. )|10 VideosCARBON AND ITS COMPOUNDS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET-19 |24 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-ATOMIC STRUCTURE-QUESTION BANK-20
- The statements below are false. For each statement, replace the underl...
Text Solution
|
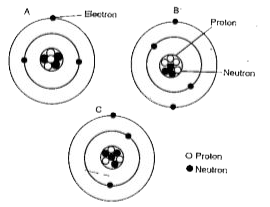
- Which diagram represents,isotopes of the same element?
Text Solution
|
- What is the atomic number for A?
Text Solution
|
- What is the mass number for B?
Text Solution
|
- Classify the following elements as metals, non metals and inert gases ...
Text Solution
|
- Use the following terms in the same sentence proton, neutron, and isot...
Text Solution
|
- Complete each of the following sentences An atom's is equal to t...
Text Solution
|
- Complete each of the following sentences by choosing the correct term ...
Text Solution
|
- Match the entries in column A with the appropriate ones in column B.
Text Solution
|
- All matter is made up of atoms. Which sentence correctly describes ato...
Text Solution
|
- What are the negatively charged particle inside an atom called? Use...
Text Solution
|
- The black circles in the model represent neutrons. What do the white c...
Text Solution
|
- The atomic number of an element is determined by
Text Solution
|
- Which of the following particles has no electric charge?
Text Solution
|
- How many protons does an atom with an atomic number of 23 and a mass n...
Text Solution
|
- Which of the following determines the identity of an element?
Text Solution
|
- Isotopes exist because atoms of the same element can have different nu...
Text Solution
|
- Which of the following pieces of equipment was used by J.J. Thomson to...
Text Solution
|
- Which one of the following is true of a neutron?
Text Solution
|
- The ratio between the neutrons in C and Si with respect to atomic mass...
Text Solution
|