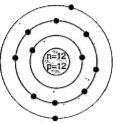
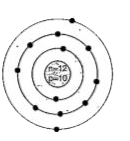
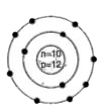
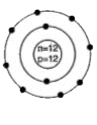
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-20 ( Answer the following questions ) |4 VideosATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET -20|23 VideosATOMIC STRUCTURE
S CHAND IIT JEE FOUNDATION|Exercise QUESTION BANK-20 (FILL IN THE BLANKS WITH APPROPRIATE WORDS TO MAKE CORRECT STATEMENTS. )|10 VideosCARBON AND ITS COMPOUNDS
S CHAND IIT JEE FOUNDATION|Exercise SELF ASSESSMENT SHEET-19 |24 Videos
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-ATOMIC STRUCTURE-QUESTION BANK-20
- Calculate the atomic mass of silicon, which occurs naturally as 92% si...
Text Solution
|
- The ion of an element has 3 positive charges. The mass number of atom ...
Text Solution
|
- How many electrons does an atom with an atomic number of 20 and a mass...
Text Solution
|
- Which of the following do not represent Bohr's model of atom correctly...
Text Solution
|
- Identify the Mg^(2+) ion from the figure where, n and p represent the ...
Text Solution
|
- Elements with valency 1 are
Text Solution
|
- Use the following terms to create a concept map atom, nucleus, prot...
Text Solution
|
- Complete the following sentences by choosing the correct term from th...
Text Solution
|
- Complete the following sentences by choosing the correct term from th...
Text Solution
|
- The statements below are false. For each statement, replace the underl...
Text Solution
|
- The statements below are false. For each statement, replace the underl...
Text Solution
|
- Compare nuclear fission with nuclear fusion.
Text Solution
|
- Imagine that a uranium nucleus splits and releases three neutrons and ...
Text Solution
|
- Which of the following is a use of radioactive material?
Text Solution
|
- Which particle both begins and is produced by a nuclear chain reaction...
Text Solution
|
- Which statement about nuclear fusion is false?
Text Solution
|
- In fission, the products have less mass than the starting materials do...
Text Solution
|
- Explain why nuclei of carbon, oxygen, and iron can be found in stars
Text Solution
|
- What are two dangers associated with nuclear fission?
Text Solution
|
- Solve the following crossword with the help of the given clues.
Text Solution
|