Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
S CHAND IIT JEE FOUNDATION-ATOMIC STRUCTURE-SELF ASSESSMENT SHEET -20
- Choose the correct options and fill in the blanks by the correct word....
Text Solution
|
- Choose the correct options and fill in the blanks by the correct word....
Text Solution
|
- Choose the correct options and fill in the blanks by the correct word....
Text Solution
|
- Choose the correct options and fill in the blanks by the correct word....
Text Solution
|
- Match the following :
Text Solution
|
- Draw diagrams representing the atomic structures of the following: O...
Text Solution
|
- Draw diagrams representing the atomic structures of the following: c...
Text Solution
|
- Which one of the following is the correct electronic configuration of ...
Text Solution
|
- What are the main postulates of Dalton's atomic theory ? What were its...
Text Solution
|
- What will be the composition of the nucleus of the atom of an element ...
Text Solution
|
- Which atom does not have any neutron in the nucleus and why?
Text Solution
|
- State the differences between atoms and ions.
Text Solution
|
- Which symbols are used to represent different Bohr's orbits?
Text Solution
|
- How does their energy change when we move outwards from the nucleus?
Text Solution
|
- Rutherford's experiment showed for the first time that the atom has
Text Solution
|
- Neutron is present in all atoms except
Text Solution
|
- The statements below are false. For each statement replace the underli...
Text Solution
|
- The statements below are false. For each statement replace the underli...
Text Solution
|
- What refinements did Bohr make to Rutherford's proposed atomic theory?
Text Solution
|
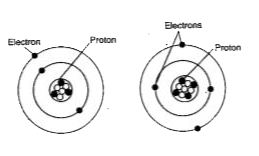
- Look at the two atomic models. Do the two atoms represent different el...
Text Solution
|