Text Solution
Verified by Experts
Topper's Solved these Questions
IS MATTER AROUND US PURE ?
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MULTIPLE CHOICE QUESTIONS-LEVEL-1)|30 VideosIS MATTER AROUND US PURE ?
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MULTIPLE CHOICE QUESTIONS-LEVEL-2)|20 VideosIS MATTER AROUND US PURE ?
MTG IIT JEE FOUNDATION|Exercise SOLVED EXAMPLES|34 VideosFOOTSTEPS TOWARDS(CBSE BOARD)
MTG IIT JEE FOUNDATION|Exercise SECTION-D|14 VideosMATTER IN OUR SURROUNDINGS
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD/HOTS CORNER|15 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-IS MATTER AROUND US PURE ?-NCERT SECTION
- Which separation techniques will you apply for the separation of the f...
Text Solution
|
- Which separation techniques will you apply for the separation of the f...
Text Solution
|
- Which separation techniques will you apply for the separation of the f...
Text Solution
|
- Which separation techniques will you apply for the separation of the f...
Text Solution
|
- Write the steps you would use for making tea. Use the words solution, ...
Text Solution
|
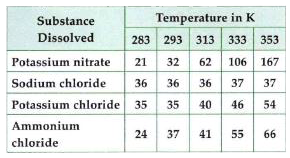
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Pragya tested the solubility of three different substances at differen...
Text Solution
|
- Explain the following by giving examples. saturated solution
Text Solution
|
- Explain the following by giving examples. pure substance
Text Solution
|
- Explain the following by giving examples. colloid
Text Solution
|
- Explain the following by giving examples. suspension
Text Solution
|
- Classify each of the following as a homogeneous or heterogeneous mixtu...
Text Solution
|
- How would you confirm that a colourless liquid given to you is pure wa...
Text Solution
|
- Which of the following materials fall in the category of a "pure subst...
Text Solution
|
- Identify the solutions among the following mixtures. (a) Soil, (b) sea...
Text Solution
|
- Which of the following will show "Tyndall effect "? (a) Salt solutio...
Text Solution
|
- Classify the following into elements, compounds and mixtures. (a) Sodi...
Text Solution
|
- Which of the following are chemical changes ? (a) Growth of a plant ...
Text Solution
|