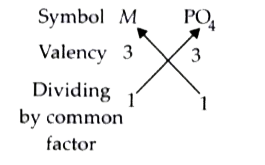
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MATCH THE FOLLOWING) |5 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (ASSERTION & REASON TYPE) |10 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise NCERT SECTION |25 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS AND MOLECULES -EXERCISE (MULTIPLE CHOICE QUESTIONS LEVEL-1)
- Molecular mass is defined as the
Text Solution
|
- The molecular formula P(2)O(5) means that
Text Solution
|
- The formula of chloride of a metal M is MCl3, then the formula of the ...
Text Solution
|
- An atom is the:
Text Solution
|
- Which of the following represents a polyatomic ion?
Text Solution
|
- Valency of silver in Ag(2)S is:
Text Solution
|
- 52 g of He contains
Text Solution
|
- Atomicity of sulphur is:
Text Solution
|
- A chemical equation is always balanced to fulfil the condition of
Text Solution
|
- Chemical formula of ferric oxide is:
Text Solution
|
- Mass of 1 mole of nitrogen atoms is:
Text Solution
|
- If the atomic mass of sodium is 23, the number of moles in 46gm of sod...
Text Solution
|
- How many molecules are present in one in one gram of hydrogen?
Text Solution
|
- In which of the following the valency of each of the constituent eleme...
Text Solution
|
- Number of moles of water present in 180 g of water will be:
Text Solution
|
- Mass of 3 moles of NaOH in grams is:
Text Solution
|
- Which one of the following statements is incorrect?
Text Solution
|
- The volume occupied by 1 mole atom of a diatomic gas at STP is
Text Solution
|
- Molecules of phosphorus and ammonia are respectively:
Text Solution
|
- Which of the following molecules has highest number of particles?
Text Solution
|