A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MATCH THE FOLLOWING) |5 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (ASSERTION & REASON TYPE) |10 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise NCERT SECTION |25 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS AND MOLECULES -EXERCISE (MULTIPLE CHOICE QUESTIONS LEVEL-1)
- Which of the following molecules has highest number of particles?
Text Solution
|
- Which one of the following parts of gases contains the same number of ...
Text Solution
|
- Weight of 6.022 xx 10^(20) atoms of silver (at. mass 108 u) is
Text Solution
|
- The number of carbon atoms in 1 g of CaCO3 is,
Text Solution
|
- In carbon disulphide (CS2), the mass of sulphur in combination with 3....
Text Solution
|
- Which one of the following statements is true?
Text Solution
|
- Two gaseous sample were analysed. One contained 1.2 g of carbon and 3....
Text Solution
|
- 20.8g of BaCI2 on reaction with 9.8g of H2SO4 produces 7.3 g of HCI an...
Text Solution
|
- The number of atoms in 4.25g of NH(3) is approximately
Text Solution
|
- A sample of phosphorus trichloride (PCl(3)) contains 1.4 moles of the ...
Text Solution
|
- The mass of one C atom is:
Text Solution
|
- What are the total number of moles of atoms in 4.32 g of Sc(NO(3))(3)?...
Text Solution
|
- The weight of a molecule of the compoundC(60)H(122) is
Text Solution
|
- In a science project, Aditya has to make a chart, illustrating various...
Text Solution
|
- The mass of few entities are mentioned in the column II. Match the two...
Text Solution
|
- Which of the following is a suitable example for illustrating the law ...
Text Solution
|
- Which of the following correctly represents 360 g of water? (i) 2 mo...
Text Solution
|
- In an experiment, 1.50 g of pure copper(II) oxide was reduced to pure ...
Text Solution
|
- A solution contains 58.5 g of common salt in 360 g of water. Calculate...
Text Solution
|
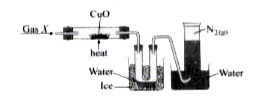
- The given figure shows the set-up to study the reaction between gas X ...
Text Solution
|