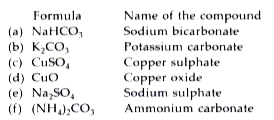Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (SUBJECTIVE PROBLEMS LONG ANSWER TYPE) |5 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (SUBJECTIVE PROBLEMS VERY SHORT ANSWER TYPE) |15 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS AND MOLECULES -EXERCISE (SUBJECTIVE PROBLEMS SHORT ANSWER TYPE)
- Carbon and oxygen react with each other in the ratio 3 : 8 by mass. Wh...
Text Solution
|
- Give the chemical formula, for each of the following acids : (a) Nit...
Text Solution
|
- Calculate the volume occupied by 2.8 g of N2 at STP.
Text Solution
|
- 8.4 g of sodium bicarbonate on reaction with 20 g of acetic acid liber...
Text Solution
|
- What is the mass of 0.5 mole of wate (H(2)O). (Atomic masses : H =...
Text Solution
|
- Calculate the number of atoms of each element present in 122.5 g of KC...
Text Solution
|
- Calculate the number of moles in 2800 mL of oxygen gas at STP.
Text Solution
|
- Calculate the number of moles in 12.044 xx 10^(23) helium atoms.
Text Solution
|
- Why does the atomic mass of an element not represent the actual mass o...
Text Solution
|
- What is the difference between the actual mass of a molecule and gram ...
Text Solution
|
- An element has fractional atomic mass. What does it indicate ?
Text Solution
|
- How many moles are present in 11.5 g of sodium?
Text Solution
|
- How many particles are represented by 0.25 mole of an element?
Text Solution
|
- Differentiate between an atom and an ion.
Text Solution
|
- Write the formula and names of compounds formed by: (a) Na^(+) and H...
Text Solution
|