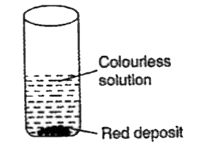
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (Match the Following)|5 VideosMATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (Assertion & Reason Type)|10 VideosMATERIALS : METALS AND NON-METALS
MTG IIT JEE FOUNDATION|Exercise EXERCISE MCQ.s (Level-1)|30 VideosFOOTSTEPS towards(CBSE Board)
MTG IIT JEE FOUNDATION|Exercise SECTION - D|14 VideosMATTER : ELEMENTS COMPOUNDS AND MIXTURES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE )|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-MATERIALS : METALS AND NON-METALS-EXERCISE MCQ.s (Level-2)
- Aluminium is a type of metal that is used to make
Text Solution
|
- The reason(s) why aluminium is preferred over copper for making overhe...
Text Solution
|
- Gravity separation method is based upon:
Text Solution
|
- A copper coin is kept immersed in a solution of silver nitrate for som...
Text Solution
|
- Which of the following pairs will give displacement reactions?
Text Solution
|
- An unknown metal X when placed in copper sulphate solution gives a red...
Text Solution
|
- In the process of welding metals like stainless steel and aluminium
Text Solution
|
- Which of the following metals on reaction with sodium hydroxide soluti...
Text Solution
|
- Match the following. Which of the following shows the correct mat...
Text Solution
|
- In which test tubes, the rusting of iron nail will take place ?
Text Solution
|
- Match the items in column I with column II
Text Solution
|
- A highly reacting element X is stored under water. It readily reacts w...
Text Solution
|
- Two elements A and B on burning in air give corresponding oxides. Oxid...
Text Solution
|
- Metallurgy is a process of extracting
Text Solution
|
- When few granules of sample X are added to a solution of copper sulpha...
Text Solution
|
- Choose the reactions which are not feasible. I. Iron+Zinc sulphateto...
Text Solution
|
- Meenu sets up an electric circuit as shown in the figure by usingg cop...
Text Solution
|
- Study the table carefully and select the appropriate option.
Text Solution
|
- Some materials like magnesium ribbon, aluminium foil, copperr wire and...
Text Solution
|
- Extraction of highly electropositve metal is done by
Text Solution
|