Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS, MOLECULES AND ATOMIC STRUCTURE-EXERCISE ( Integer/Numerical Value Type)
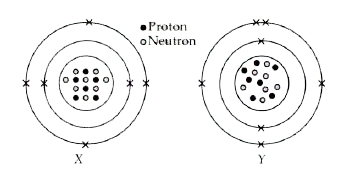
- The given diagrams show the atomic structures of elements X and Y. ...
Text Solution
|
- Mass of CO(2) (in gram) which have 2.55 L volume at S.T.P. is
Text Solution
|
- Number of diatomic molecules among the following is hydrogen molecule,...
Text Solution
|
- The total number of molecules present in 6.6 g of CO(2) and 4.8 g of S...
Text Solution
|
- The valency of an element with atomic number 15 is
Text Solution
|
- The total number of electrons present in the M-shell of sulphur is
Text Solution
|