Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS, MOLECULES AND ATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise EXERCISE (Multiple Choice Questions)|50 VideosATOMS, MOLECULES AND ATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise EXERCISE (Match the following)|6 VideosATOMS, MOLECULES AND ATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise EXERCISE ( Integer/Numerical Value Type)|5 VideosCOAL AND PETROLEUM
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD/HOTS CORNER|10 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS, MOLECULES AND ATOMIC STRUCTURE-SOLVED EXAMPLES
- Calculate the number of moles in 162 g of Al,
Text Solution
|
- Calculate the number of moles in 69 g of Na. given : Atomic masses : A...
Text Solution
|
- Calculate the number of molecules in each of the following : 124 g o...
Text Solution
|
- Calculate the number of molecules in each of the following : 11 g of...
Text Solution
|
- Calculate the weight of carbon monoxide having the same number of oxyg...
Text Solution
|
- Calculate the number of atoms of each element in 3.42 g of sucrose (C(...
Text Solution
|
- Calculate the mass of a single atom of sulphur and a single molecule o...
Text Solution
|
- Which has more number of atoms: 115 g of sodium or 168 g of iron? (Giv...
Text Solution
|
- Calculate the number of gram atoms and gram molecules in 2.54 mg of io...
Text Solution
|
- 10^(22) atoms of an element 'X' are found to have a mass of 531 mg. Ca...
Text Solution
|
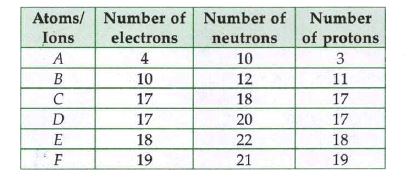
- Elements from A to F have in them the distribution of electrons, neutr...
Text Solution
|
- Elements from A to F have in them the distribution of electrons, neutr...
Text Solution
|
- Elements from A to F have in them the distribution of electrons, neutr...
Text Solution
|
- Elements from A to F have in them the distribution of electrons, neutr...
Text Solution
|
- Y is the ion of an element X. Y contains 13 protons, 14 neutrons and 1...
Text Solution
|
- Y is the ion of an element X. Y contains 13 protons, 14 neutrons and 1...
Text Solution
|
- Y is the ion of an element X. Y contains 13 protons, 14 neutrons and 1...
Text Solution
|