Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS towards(CBSE Board)-SECTION - D
- How will you prove that air is necessary for combustion
Text Solution
|
- How will you prove that the non-luminous zone of a candle flame is t...
Text Solution
|
- What is a fire extinguisher? Explain the construction and working of a...
Text Solution
|
- How is CNG obtained? What is its full form? Write the advantages of us...
Text Solution
|
- Define galvanisation.
Text Solution
|
- Magnesium powder was added to two different salt solutions in beakers ...
Text Solution
|
- Magnesium powder was added to two different salt solutions in beakers ...
Text Solution
|
- Magnesium powder was added to two different salt solutions in beakers ...
Text Solution
|
- Magnesium powder was added to two different salt solutions in beakers ...
Text Solution
|
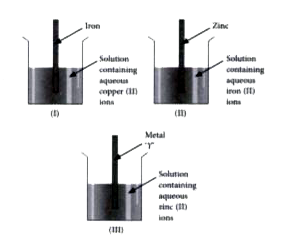
- Radha arranged three set-ups as shown in the following figures. She ob...
Text Solution
|
- Radha arranged three set-ups as shown in the following figures. She ob...
Text Solution
|
- Radha arranged three set-ups as shown in the following figures. She ob...
Text Solution
|
- Radha arranged three set-ups as shown in the following figures. She ob...
Text Solution
|
- Radha arranged three set-ups as shown in the following figures. She ob...
Text Solution
|